2. Brief overview of cleavage#
This paragraph is in particular inspired by [2], which can be referred to for more microstructural details in particular.
Cleavage is a mode of rupture whose main mechanism is the separation of atomic planes, with virtually no deformation. It is the main mechanism of brittle failure in metals, especially in the case of weak crystalline symmetries such as centered cubic or hexagonal ones. This failure mode being in competition with plastic deformation, it is facilitated by a low temperature (the deformation mechanisms are then less activated). This leads to the existence of a fragile (at low temperature) -ductile (at higher temperature) transition. Among the models that are defined here, the two deterministic methods are currently being tested in the case of the transition zone in order to predict the risk of cleavage. However, this use requires caution; to our knowledge, no method can currently be considered reliable for predicting the risk of cleavage in the transition zone.
The morphology of the surfaces broken by cleavage corresponds to transgranular propagation. It can be easily observed by microscopy, as shown in Figure. It is frequently characterized by the presence of lines parallel to the direction of propagation, called rivers.
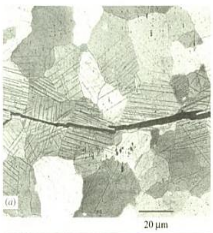
Figure 2-1: Microscopic facies of cleavage
Fracture by cleavage, like other methods of failure by cracking, theoretically distinguishes between two mechanisms: initiation and propagation. Initiation corresponds to the development of a micro-crack inside the healthy metal; it is generally accepted that this step requires a fairly low prior plastic deformation, which causes a stack of dislocations and a singularity of the stresses, and the achievement of a breaking stress limit, and the achievement of a breaking stress limit, now noted \({\sigma }_{c}\). The propagation of cleavage cracks is unstable, has a high speed (of the order of 40% of the speed of sound in metal), and must satisfy an energy criterion such as Griffith [3].
Since cleavage is not accompanied by significant deformations, it does not require a great deal of energy, unlike ductile rupture. This is why the Charpy test, measuring resilience (energy required for the impact of a standardized test piece to break), makes it possible to distinguish between these two types of failure for the same material (see Figure). At low temperatures, the energy required for rupture is low: the mode of rupture is cleavage. At high temperature, the energy required for rupture is high: rupture occurs by tearing. The limit temperature between these two phenomena is called the transition temperature; there are several definitions, including those based on a given energy level (we speak for example of \({T}_{\mathrm{k65}}\), which would be equal here to approximately \(\mathrm{-}20°C\)).
Irradiation of metals induces an increase in the elastic limit and makes it more difficult to stack dislocations, and in fine promotes cleavage: the transition temperature of an irradiated material is higher than that of the same non-irradiated material. In the transition zone, i.e. the temperature range close to this transition temperature, breakage can occur by cleavage, by tearing, or by tearing over a finite length followed by cleavage. The prediction of cleavage in transition zones remains a field of research: even if methodologies are proposed here, they still require validation and should not be seen as « turnkey » methods.
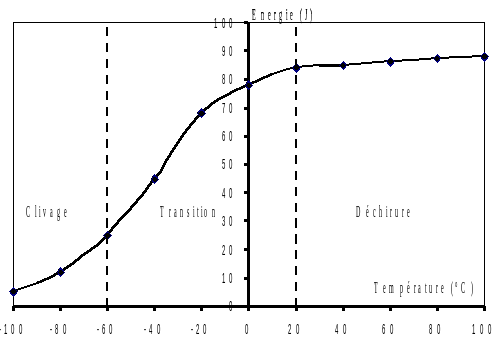
Figure 2-2: Fragile Ductile transition curve by Charpy test